What is DLC, Also Known as Diamond-Like-Carbon Coating?
In materials and finishes, Diamond-Like Carbon (DLC), also called amorphous carbon (a-C), isn't one coating but an entire category of coating materials. DLC has properties that change depending on the bonding of the carbon atoms and the presence or lack of other materials in the coatings. These materials include hydrogen, silicon, or metals. Generally, DLC is known for:
- High hardness
- Low friction
- Wear resistance
- Corrosion resistance
- A striking dark gray color
- Chemical inertness
We can adjust these properties for different applications, optimizing them to specific needs for improving components' appearance, performance, and durability in many industries (if you are interested in using DLC on firearms, visit our Firearms coating page). Let’s back up and talk about what makes the carbon in DLC so unique.
Graphite vs. diamond: what is DLC coating?
Carbon (atomic symbol C) has some exciting and valuable properties. In one form, it makes up graphite, a very soft, black, conductive material made of layers that slide easily relative to one another. Pencil ‘leads’ are made of graphite because it glides easily and is soft enough to leave behind residue on paper.
On the other hand, diamonds are also made of carbon. Diamond is clear, electrically insulating, and extremely hard: the hardest naturally occurring material on earth. We use small diamonds for cutting other hard materials, and large ones are considered precious gems. Diamonds have properties that are nothing like graphite. Both materials are made of the same element, and the only difference between graphite and diamond is the bonding and structure of the carbon atoms in each.
Want to know more about Diamond-Like Carbon (DLC) coating? Contact us today!
Atomic bonding and structure
Carbon is tetravalent, meaning it has four electrons available for molecular bonds with other atoms. In graphite, only three electrons form bonds with other carbon atoms, forming a two-dimensional plane of hexagonal shapes via sp2 bonding. These individual layers are called graphene. The bonds within these layers are strong, but when additional layers are added, the bonding between the layers is weak, so the sheets can easily slide past one another. That makes graphite soft and gives it some of its other properties.
Diamond, on the other hand, has all four electrons forming molecular bonds with other carbon atoms. These carbon atoms form a three-dimensional tetrahedral shape via sp3 bonding. The carbon atoms pack together tightly inside the diamond crystal, and the bonds are quite strong. The result is the hard, clear crystal we are familiar with, formed deep within the earth or in a laboratory under extremely high pressures.
Like graphite and diamond, DLC is made of carbon, but it has a combination of sp2 and sp3 bonds. As a result, a DLC coating has a combination of the properties of both diamond and graphite. For example, it can be very hard but also have very low friction. In the coating process, we tune the properties by changing process conditions and energy to change the bonding. As sp3 bonding increases, the coating becomes harder and more like a diamond, or “diamond-like.” Pure carbon with mostly sp3 bonding is called tetragonal amorphous carbon (t-aC).
Doping: add other materials for different coating properties
Adding other elements to the diamond-like carbon is called "doping." Just like impurities in diamonds can change properties such as color, adding other materials can adjust the properties of these coatings, making it possible to tune the color, conductivity, hardness, wear resistance, friction, and many other properties.
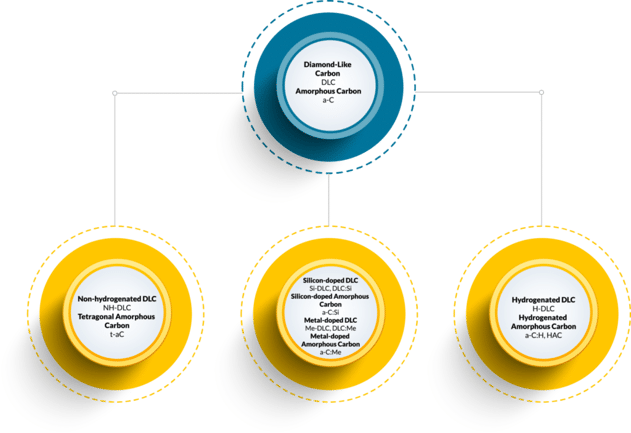
Alphabet Soup: Sorting out the DLC acronyms
There are many different acronyms and abbreviations for different types of DLC coatings, and there are often several ways to refer to the same kind of coating. This is where DLC coatings can get confusing, so I’ve broken it down in an easy-to-understand chart.
The chart shows just a few examples of naming conventions. For DLCs, the metal is often specified using its atomic abbreviation in place of the ‘Me’ (e.g., W-DLC or a-C:Ti). The types can also be combined, for example, metal-doped hydrogenated DLC (Me-C:H). Often, a DLC name includes a number that represents the atomic percentage of hydrogen or metal doping. The variations continue, but it doesn’t need to be that complicated. Now, the next time someone mentions DLC, you will know the answer to: “What is diamond-like carbon?”
If you think one of these Diamond-Like Carbon coatings might suit you and your product, contact Vapor Technologies, Inc. We can help you cut through the mystique of all the variations and develop a coating solution specific to your product needs. We often have people ask us, “What is DLC coating?” And we hope we’ve provided the answers you need. You can also access more information about VT-Diamond coatings on our DLC coatings page.
Recent posts

Our DLC Coating Machines Produce Highly Targeted Functional DLC Coatings

Your Top 3 DLC Coating Questions Answered